Abstract
Background: Extranodal Natural killer (NK) /T-cell lymphoma, nasal type (NNKTL), a unique and aggressive neoplasm, is commonly observed in East Asia and Latin America. Even at early stages, NNKTL has a poor clinical outcome because intrinsic chemotherapy resistance, rapid local disease progression and distant metastasis. Despite the use of high dose radiation therapy with or without multiagent chemotherapy regimens, NNKTL has a high rate of relapse (25-40%). Moreover, the estimated 5-year survival on relapsed NNKTL is 52.3%. The identification and validation of readily available biomarkers that predict treatment outcomes can aid in the use of consolidation cellular therapy (i.e. allogeneic bone marrow transplant) and tailored surveillance programs. Elevated serum levels of soluble intercellular adhesion molecule-1 (sICAM-1) had been associated with inferior outcomes in non-Hodgkin lymphoma, but their utility in NNKTL remains unidentified. Here, we examined sICAM-1 levels in patients of early-stage NNKTL and its correlation with clinical characteristics, response to the therapy and clinical outcomes.
Material and Methods: This was a retrospective observation study conducted in patients diagnosed as Stage I/II NNKTL in the upper aerodigestive tract treated at Shanghai Cancer Hospital, Shanghai, China and for which biospecimens collected at diagnosis and at the end of therapy (EOT) (frozen serum) were available. The study was approved by the IRB (Protocol number). Baseline and EOT sICAM-1 levels were measured by ELISA (R&D systems, Minneapolis, MN, sensitivity =0.35ng/ml) according to the manufacture' s protocol. Differences of sICAM-1 expression between baseline and EOT were analyzed by paired t-test. Difference of sICAM-1 between complete response and non-complete response groups were determined by Mann-Whitney U-test. Comparing sICAM-1 among different clinical characteristics was determined by Chi square and Fisher's test. Differences in overall survival (OS) and progression-free survival (PFS) between patients with high and low sICAM-1 levels were analyzed by the Kaplan-Meier method.
Results:
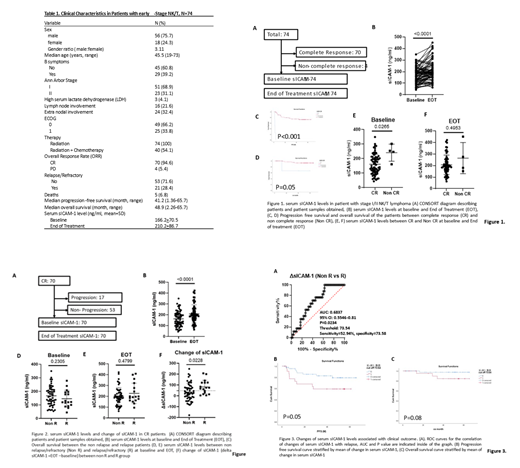
A total of 74 patients were included in the study (18F/56M), the median age of the patients was 45.5 (19-73). Patients were treated with radiation therapy alone (N=34), or combined modality therapy (N=40). As previously noted in other studies, achievement of a complete response at the end of therapy was associated with a better PFS and OS (P<0.001). The median sICAM-1 at the EOT was higher (210.2+86.7ng/ml) when compared to baseline levels (166.2+70.5ng/ml, p<0.001). Of interest the sICAM-1 levels at baseline were higher among patients that failed to complete a complete response (CR) (241.2+58.7ng/ml vs 162.6+69.3ng/ml, p= 0.0265).
The net change (EOT minus Baseline) of sICAM-1 levels (ΔsICAM-1) was significantly higher is patients with relapse disease (81.4+63.1 vs 33.5+81.2, p<0.023). Furthermore, higher ΔsICAM-1 was associated with shorter PFS (40m vs 56m, P=0.05) and a trend towards a poor overall survival although p value did not reach statistically difference.
Conclusion: Higher sICAM-1 levels at diagnosis were associated with primary treatment failure in early stage NNKTL. In addition, an elevated ΔsICAM-1 in patients achieving a complete response to first like therapy were associated with relapse disease and shorter PFS. Together, our data suggests that sICAM-1 may be used as serological biomarker to monitor and predict clinical outcome of early stage NNKTL patients.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal